The U.S. Food and Drug Administration(FDA) has approved the use of blood plasma extracted from survivors of COVID-19 in the treatment of the disease.
SEE ALSO: FDA Approves Remdesivir As Emergency Treatment For Coronavirus
FDA said in statement on Sunday that the move was part of its ongoing efforts to fight COVID-19.
“Based on scientific evidence available, the FDA concluded, as outlined in its decision memorandum, this product may be effective in treating COVID-19.
“It also agreed that the known and potential benefits of the product outweigh the known and potential risks of the product,” it said
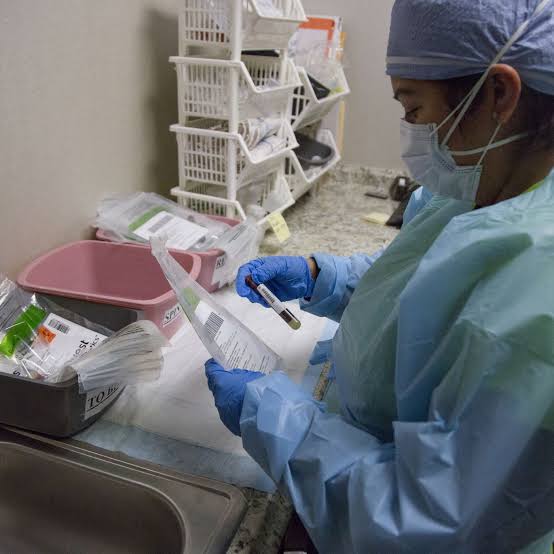
The treatment involves taking blood rich in antibody from recovered COVID-19 patients, otherwise known as convalescent plasma, and providing it to those infected.
FDA Commissioner, Mr Stephen Hahn, said on Sunday that the therapy had shown 35 per cent success rate.
However, reports say evidence remains inconclusive about its effectiveness and appropriate dosage.
“I am committed to releasing safe and potentially helpful treatments for COVID-19 as quickly as possible in order to save lives.
“We’re encouraged by the early promising data that we’ve seen about convalescent plasma,” he said.
Hahn, however, noted that the treatment still needed to undergo randomised clinical trials to determine its safety and effectiveness.
The trials, which started in New York and some other states since April, had suffered delays and issues with finding volunteers.
On Saturday, President Donald Trump lashed out at the FDA for hindering approval of coronavirus vaccines and therapeutics for political reasons.
Sunday’s announcement came a day before the Republican National Convention where Trump would be formally nominated as the party’s candidate for the Nov. 3 presidential election.
The president took credit for the authorisation during his evening news conference on Sunday, saying it would “dramatically expand access to this treatment.”
“We are years ahead of approvals if we went by the speed of past administrations. And that includes vaccines,” Trump said.



 Premier League
Premier League