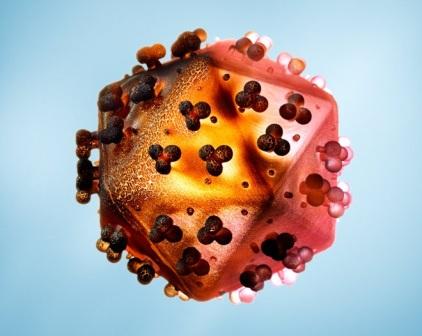
The experimental drug for HIV/AIDS by British pharmaceutical company, GlaxoSmithkline (GSK) referred to as dolutegravir has received priority status by the United States Food and Drug Administration.
Drugs are given priory status when the FDA believes they could possibly provide notable improvement over current treatments.
Dolutegravir is a drug which has been thought of by manufacturing analysts as a potential multibillion-dollar-a-year seller and the decision on whether to approve the HIV/AIDS drug will be made by August 17th, says sources at its maker, GSK.
The drug is meant to be taken once a day and belongs to a new class of medicines called integrase inhibitors, which stops the virus that causes AIDS from entering cells.
The treatment is owned by Viiv Healthcare, a joint venture between GSK and Pfizer set up in 2009 when they decided to combine their HIV/AIDS drug businesses into one company. It was 85% owned by GSK and 15% owned by Pfizer at formation.
The novel medication has shown positive results in clinical studies, which pushed GSK to redraw its contract with the Japanese drug maker Shionogi.
The agreement now states that Shionogi has a 10% stake in Viiv and will receive its shared rights to the new drug. It also states that GSK owns 76.5% of the joint venture, and 13.5% is owned by Pfizer.
Dolutegravir will be a tough competitor to Gilead Sciences’ drugs, the most common treatments for HIV, industry specialists said.



 Premier League
Premier League