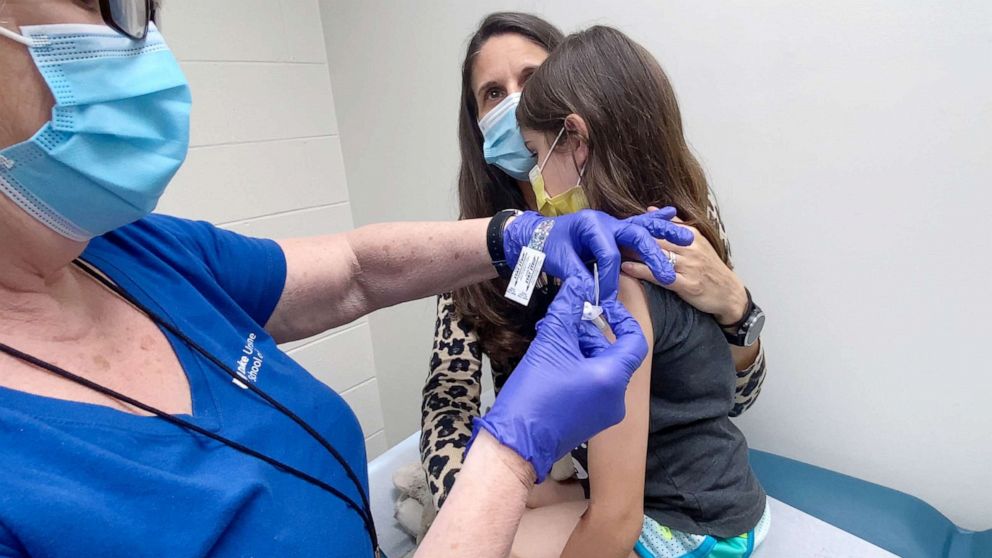
The Food and Drug Administration on Monday granted Pfizer’s request to expand the use of its COVID-19 vaccine to adolescents between the ages of 12-15.
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” acting FDA Commissioner Janet Woodcock said in a statement.
“Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
Pfizer reported that 2,260 people, ages 12 to 15, were subjected to clinical trials where participants were either given two doses of the vaccine or a placebo three weeks apart. It was discovered that the vaccine was highly effective at preventing symptomatic illness, while side effects mirrored what was previously seen in the 16- to 25-year-old age group. The only difference was that fevers appeared to be a slightly more common occurrence in 12- to 15-year-olds.
Read also: I’ll run for president in 2023 if insecurity doesn’t end – Doyin Okupe
In March, Pfizer and BioNTech began trials on children aged 5 to 11, but the scope of their study expanded last month to include participants as young as two years old. The company is expected to apply for emergency authorization use with the FDA in September.
The move could be a game-changer for getting students back into the classroom, as some parents have expressed interest in vaccinating their children before resuming in-person schooling. Middle and high schools have particularly struggled with physical reopenings because it is more difficult to cohort students and staff into groups that stay together throughout the day.



 Premier League
Premier League