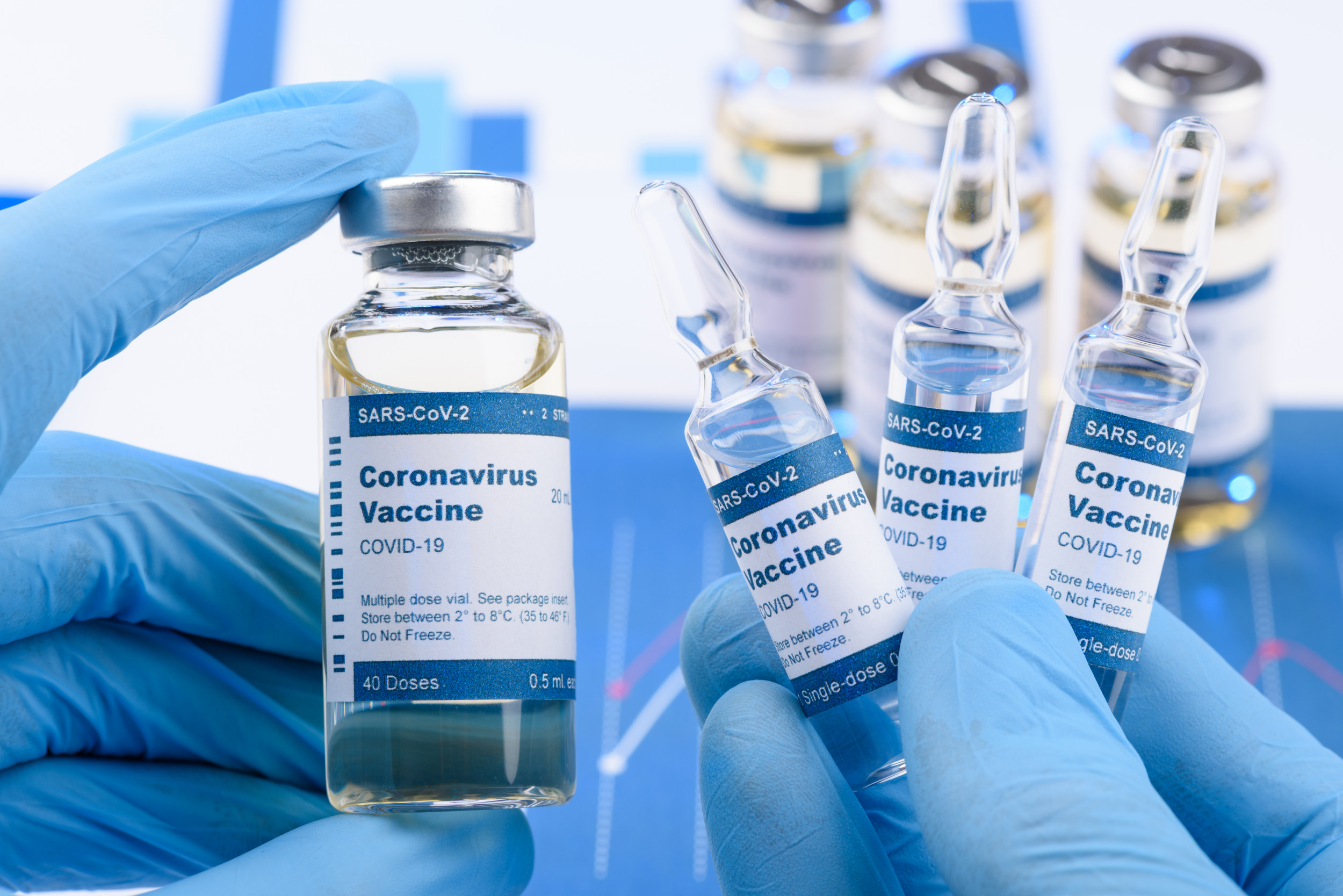
Top U.S. infectious disease expert, Dr Anthony Fauci, has said that the first doses of a successful COVID-19 vaccine would be available to some hardest-hit Americans by late December or early January.
Fauci, who is the Director of the National Institute of Allergy and Infectious Diseases, stated this during a live Facebook chat on Thursday.
He said Americans would know in December whether or not they would have a safe and effective vaccine, citing leading vaccine developers, Pfizer and Moderna.
The comments came as the country reported no fewer than 74,000 new cases of COVID-19 on Thursday.
The U.S. coronavirus caseload currently stands at no fewer than 8.9 million, including 228,194 deaths since the outbreak of the pandemic.
READ ALSO: Nigeria Can’t Afford Another Lockdown – Buhari
Fauci said the first preliminary COVID-19 vaccine trial results should be out within the next few weeks.
According to him, the first vaccine doses will likely be made available to individuals considered most in need “by the end of December or the beginning of January.”
He was, however, quick to warn that it would take time for normalcy to return to the country even with an effective vaccine.
The U.S. Government has since pre-ordered millions of promising vaccine candidates with Pfizer and Moderna, whose final stage of clinical testing began in late July.
Reports quoted Moderna as saying on Thursday that it was “on track to deliver interim data from its large, late-stage trial next month”.
Pfizer was expected to release its interim results in October but has missed the target, and expectations are that it would do so in early or mid-November.
After release, the data will then be reviewed by the U.S. Food and Drug Administration and the Center for Disease Control and Prevention. (NAN)



 Premier League
Premier League