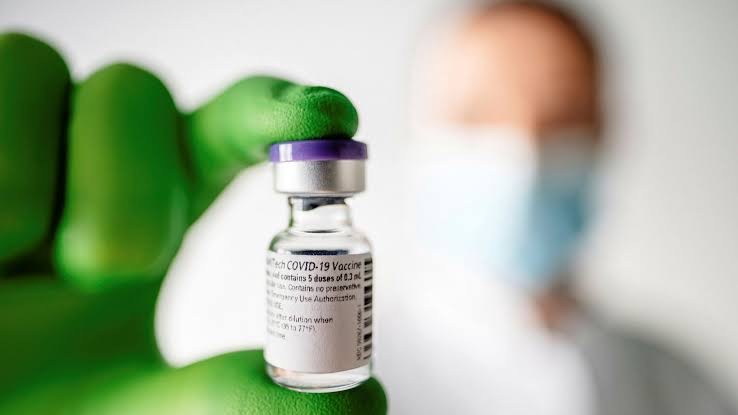
Pfizer and BioNTech on Wednesday became the first vaccine developers in the race to alleviate the COVID-19 pandemic to get regulatory approval after completing all stages of clinical testing.
SEE ALSO: Donald Trump Gives Approval for TikTok, Oracle Deal
The companies a U.S. pharma giant and its once relatively unknown partner in the German city of Mainz announced that Britain’s regulatory authority for pharmaceuticals had issued emergency use approval for their vaccine.
The go-ahead from the Medicines and Healthcare products Regulatory Agency (MHRA) paves the way for Pfizer and BioNTech to begin delivering doses immediately to the country, they said.
The companies have already signed an agreement to supply a total of 40 million doses to Britain over the course of 2020 and 2021.
Regulatory approval is still pending in the European Union and the United States, after requests were submitted to the relevant authorities there earlier this week.
“This constitutes the first emergency use authorisation following a worldwide phase-3 trial of a vaccine to help fight the pandemic,’’ Pfizer and BioNTech said in a statement.
Pfizer chief executive hailed the British regulator’s emergency approval as “a historic moment in the fight against CO|VID-19.’’
The vaccine has been found to be 95 per cent effective against COVID-19.
In a final analysis of clinical trials released in November, based on tests involving tens of thousands of volunteers, Pfizer and BioNTech said it worked almost equally well across different demographic age groups and that no safety concerns were noted.
The drug protects against the COVID-19 lung disease caused by the novel Coronavirus. It remains unclear whether the vaccine also prevents the carrier from passing on the virus.
BioNTech chief executive Ugur Sahin said the vaccine’s approval for emergency use in Britain “will reduce the number of people in the high-risk population being hospitalised’’ there.
Britain has counted one of the world’s highest national death tolls in the pandemic, with almost 60,000 people having died after catching the virus, according to the Department of Health.
A spokesperson for the ministry said that the government had accepted the MHRA’s recommendation.
“This follows months of rigorous clinical trials and a thorough analysis of the data by experts at the MHRA who have concluded that the vaccine has met its strict standards of safety, quality and effectiveness,’’ the spokesperson added.
The BioNTech/Pfizer vaccine is also the first vaccine with regulatory approval to use mRNA technology, which draws on the virus’ genetic code to train the body’s immune system to create antibodies against it.
READ: UK Agrees Deals For 90m Doses Of Potential Coronavirus Vaccines
In Russia, China and most recently Bahrain, vaccines have already been made available and some of the population has received the jab.
However, it remains unclear how effective these vaccines are and what side effects they could have.